The first gene therapy improves survival rates of young SMA patients
 With the advancement of medical science and technology, gene therapy has made the impossible possible, and diseases that have left people powerless have seen a turnaround. In the past, patients with spinal muscular atrophy (SMA) had to undergo life-long treatment, including spinal injections in hospitals every three to four months or daily oral medication. Now, the Hospital Authority (HA) has introduced gene therapy, significantly improving the survival rate of SMA patients with the one-time intravenous administration of a drug.
With the advancement of medical science and technology, gene therapy has made the impossible possible, and diseases that have left people powerless have seen a turnaround. In the past, patients with spinal muscular atrophy (SMA) had to undergo life-long treatment, including spinal injections in hospitals every three to four months or daily oral medication. Now, the Hospital Authority (HA) has introduced gene therapy, significantly improving the survival rate of SMA patients with the one-time intravenous administration of a drug.
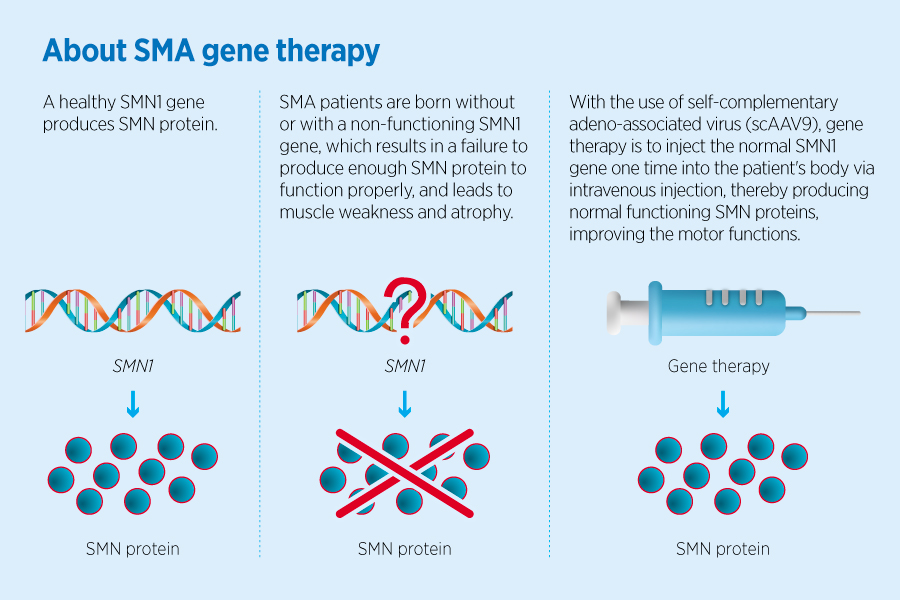
“Having to go to the hospital for injections every few months is a traumatic experience for a child. The infusion of the one-time gene therapy takes only an hour. It can eliminate the inconvenience of long-term treatment and bring new hope to the patient and the whole family,” explains HA Chief Pharmacist Dr Benjamin Lee. Patients with SMA are unable to produce enough functioning proteins because of genetic defects which result in the loss of motor muscle functions. The new gene therapy, Onasemnogene abeparvovec, involves injecting a harmless viral vector carrying the corresponding genes into the patient’s body, which allows the genes to perform their basic functions and compensates for congenital deficiencies so that patients can maintain their muscle functions normally and avoid the need to use ventilator. On-going clinical data shows that the effects of the new drug last for up to 7.5 years after it is administered.
HA Cluster Services Director Dr Simon Tang says, “once the HA knew that gene therapy could benefit some SMA patients, we negotiated with the pharmaceutical company to provide one dose of the new drug free of charge for clinical treatment. In parallel, the SMA expert panel formulated clinical guidelines for the new drug, and we also successfully applied for the drug to be included in the ultra-expensive drug category of the Community Care Fund (CCF) for subsidising families of SMA patients with financial needs.”
 The team was facing challenges in selecting the first patient for treatment. “At that time, the new drug had yet to be included in the CCF and we only had one injection. We are grateful to the SMA expert panel for selecting the patient based on clinical evidence,” Dr Tang recalls. “Thankfully, the new drug has now been covered by the CCF, so in future any SMA children who meet the clinical guidelines for the new drug will be able to receive it.”
The team was facing challenges in selecting the first patient for treatment. “At that time, the new drug had yet to be included in the CCF and we only had one injection. We are grateful to the SMA expert panel for selecting the patient based on clinical evidence,” Dr Tang recalls. “Thankfully, the new drug has now been covered by the CCF, so in future any SMA children who meet the clinical guidelines for the new drug will be able to receive it.”
A team of dedicated experts on standby
Preparations for the gene therapy were also well-planned. A multi-disciplinary team of more than 30 professionals is on standby to provide gene therapy, and the hospital specifications also meet the necessary requirements, says Hospital Chief Executive of Hong Kong Children’s Hospital Dr Lee Tsz-leung (below photo). Preparations include arranging a designated biological safety cabinet, along with the well planning and training by staff of pharmacy department on how to handle, store, and thaw the injectable drug, and ensuring aseptic preparation of the drug infusion. As side effects such as fever and vomiting may occur after the injection, the medical team provided careful post-treatment care, and closely monitored the baby’s liver enzymes and blood test results. Physiotherapists, occupational therapists, and speech therapists then regularly assessed the baby’s progress.“This is a valuable learning opportunity as we will be caring for more children receiving gene therapy in the future,” says Dr Lee. “We must be fully prepared and meet the international standard from the outset. Our medical team is greatly encouraged by the improvement of conditions of the baby.”